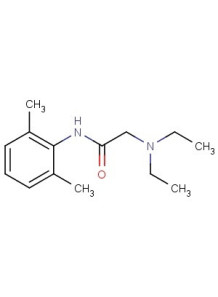
Lidocaine (Base)
- Product Code: 8391
Used as an anesthetic, local anesthesia.
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
| Test Name | Specification |
|---|---|
| Appearance | White to light yellow crystalline powder |
| Solubility | Very soluble in ethanol and in chloroform, freely soluble in benzene and in ethyl ether, practically insoluble in water |
| IR | The infrared absorption spectrum of the sample shall be consistent with that of the reference substance |
| HPLC | The retention time of the major peak of the sample solution corresponds to that of the standard solution, as obtained in the Assay |
| Chloride | NMT 0.0035% |
| Sulfate | NMT 0.1% |
| Water | NMT 0.50% |
| Residue on Ignition | NMT 0.10% |
| Impurity A | NMT 0.010% |
| Impurity H | NMT 0.10% |
| Unspecified single impurity | NMT 0.10% |
| Total impurities | NMT 0.50% |
| Diethylamine | NMT 0.032% |
| Toluene | NMT 0.089% |
| Assay | NLT 97.5% and NMT 102.5% of C₁₄H₂₂N₂O, calculated on the anhydrous basis |
This product Can only be sold to hospitals, clinics, pharmaceutical factories, pharmacists, and doctors for use in research. The buyer must submit the following documents: Natural person: Medical or pharmacist professional certification document. Legal entity: Document to receive information about the drug production location. or hospital certification document (Clinic/Hospital)
Lidocaine (base type, not HCL) used as an anesthetic, local anesthesia.
Soluble in ethanol
Usage: Use only in medicine Not allowed for use in cosmetics.
Usage rate: Not more than 2%
Product characteristics: white powder
Storage: If you want to keep it for the long term Store in the refrigerator. Do not expose to sunlight or heat. Seal the lid tightly. Shelf life is at least 2 years.
Chemical Name : Lidocaine (BP2008/USP30)
Be the first to review this product :-)
Recommend Lab-Service
| Lab Service | Price |
|---|

Used as an anesthetic, local anesthesia.
This product Can only be sold to hospitals, clinics, pharmaceutical factories, pharmacists, and doctors for use in research. The buyer must submit the following documents: Natural person: Medical or pharmacist professional certification document. Legal entity: Document to receive information about the drug production location. or hospital certification document (Clinic/Hospital)
Lidocaine (base type, not HCL) used as an anesthetic, local anesthesia.
Soluble in ethanol
Usage: Use only in medicine Not allowed for use in cosmetics.
Usage rate: Not more than 2%
Product characteristics: white powder
Storage: If you want to keep it for the long term Store in the refrigerator. Do not expose to sunlight or heat. Seal the lid tightly. Shelf life is at least 2 years.
Chemical Name : Lidocaine (BP2008/USP30)
| Mechanism | - |
| Appearance | - |
| Longevity | - |
| Strength | - |
| Storage | - |
| Shelf Life | - |
| Allergen(s) | - |
| Dosage (Range) | - |
| Recommended Dosage | - |
| Dosage (Per Day) | - |
| Recommended Dosage (Per Day) | - |
| Mix Method | - |
| Heat Resistance | - |
| Stable in pH range | - |
| Solubility | - |
| Product Types | - |
| INCI | - |
Cart
No products