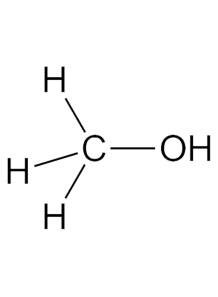
Methanol (99.9%)
- Product Code: 126210
often called wood alcohol, is the simplest alcohol with the chemical formula CH₃OH. It is a colorless, volatile, and flammable liquid at room temperature, with a characteristic faintly sweet odor
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
Methanol, often called wood alcohol, is the simplest alcohol with the chemical formula CH₃OH. It is a colorless, volatile, and flammable liquid at room temperature, with a characteristic faintly sweet odor. Methanol is produced both naturally and synthetically, and it has numerous industrial and commercial applications.
Key Properties:
- Chemical Formula: CH₃OH
- Molecular Weight: 32.04 g/mol
- Physical State: Liquid at room temperature
- Boiling Point: Approximately 64.7 °C (148.5 °F)
- Solubility: Completely miscible with water, ethanol, ether, and many other organic solvents
Production:
Historically, methanol was produced by the destructive distillation of wood, giving rise to the name “wood alcohol.” Today, it is predominantly synthesized from syn-gas (a mixture of carbon monoxide and hydrogen) using a copper-based catalyst. Another emerging method involves converting methane (the primary component of natural gas) into methanol, representing a key component in natural gas-to-liquids (GTL) technologies.
Uses and Applications:
- Chemical Feedstock: Methanol is a crucial starting material for manufacturing a wide range of chemicals, including formaldehyde, acetic acid, methyl tertiary-butyl ether (MTBE), and various plastics, resins, and adhesives.
- Fuel and Energy Applications: Methanol can be blended with gasoline to create high-octane fuel blends, and it’s used as a fuel in specialized racing engines. In addition, there’s growing interest in using methanol as a hydrogen carrier for fuel cells and for powering direct methanol fuel cells (DMFCs).
- Solvent and Extraction Agent: Thanks to its polarity and ability to dissolve a broad range of substances, methanol is commonly used as a solvent in laboratories and industrial processes.
- Denaturant for Ethanol: Methanol is frequently added to ethanol to make it undrinkable, a process known as denaturing, thus avoiding beverage alcohol taxes.
Be the first to review this product :-)
Recommend Lab-Service
| Lab Service | Price |
|---|
often called wood alcohol, is the simplest alcohol with the chemical formula CH₃OH. It is a colorless, volatile, and flammable liquid at room temperature, with a characteristic faintly sweet odor
Methanol, often called wood alcohol, is the simplest alcohol with the chemical formula CH₃OH. It is a colorless, volatile, and flammable liquid at room temperature, with a characteristic faintly sweet odor. Methanol is produced both naturally and synthetically, and it has numerous industrial and commercial applications.
Key Properties:
- Chemical Formula: CH₃OH
- Molecular Weight: 32.04 g/mol
- Physical State: Liquid at room temperature
- Boiling Point: Approximately 64.7 °C (148.5 °F)
- Solubility: Completely miscible with water, ethanol, ether, and many other organic solvents
Production:
Historically, methanol was produced by the destructive distillation of wood, giving rise to the name “wood alcohol.” Today, it is predominantly synthesized from syn-gas (a mixture of carbon monoxide and hydrogen) using a copper-based catalyst. Another emerging method involves converting methane (the primary component of natural gas) into methanol, representing a key component in natural gas-to-liquids (GTL) technologies.
Uses and Applications:
- Chemical Feedstock: Methanol is a crucial starting material for manufacturing a wide range of chemicals, including formaldehyde, acetic acid, methyl tertiary-butyl ether (MTBE), and various plastics, resins, and adhesives.
- Fuel and Energy Applications: Methanol can be blended with gasoline to create high-octane fuel blends, and it’s used as a fuel in specialized racing engines. In addition, there’s growing interest in using methanol as a hydrogen carrier for fuel cells and for powering direct methanol fuel cells (DMFCs).
- Solvent and Extraction Agent: Thanks to its polarity and ability to dissolve a broad range of substances, methanol is commonly used as a solvent in laboratories and industrial processes.
- Denaturant for Ethanol: Methanol is frequently added to ethanol to make it undrinkable, a process known as denaturing, thus avoiding beverage alcohol taxes.
| Mechanism | - |
| Appearance | - |
| Longevity | - |
| Strength | - |
| Storage | - |
| Shelf Life | - |
| Allergen(s) | - |
| Dosage (Range) | - |
| Recommended Dosage | - |
| Dosage (Per Day) | - |
| Recommended Dosage (Per Day) | - |
| Mix Method | - |
| Heat Resistance | - |
| Stable in pH range | - |
| Solubility | - |
| Product Types | - |
| INCI | - |
Cart
No products