Polyacrylic Resin E100 (2:1:1) (e.q. Eudragit E100) (Tablet)
- Product Code: 35693
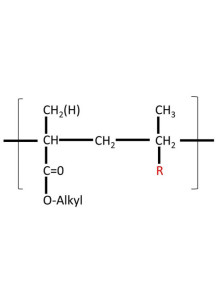
a cationic copolymer based on dimethylaminoethyl methacrylate, butyl methacrylate, and methyl methacrylate with a ratio of 2:1:1
Tablet Type
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
- -
Polyacrylic Resin E100 is a cationic copolymer mainly composed of dimethylaminoethyl methacrylate, butyl methacrylate, and methyl methacrylate with a ratio of 2:1:1. This polymer is primarily known for its mucoadhesive properties, making it suitable for various pharmaceutical formulations intended for localized drug delivery.
Here are some key features and applications of Polyacrylic Resin E100:
Mucoadhesion: Polyacrylic Resin E100 exhibits a positive charge at physiological pH, allowing it to adhere strongly to mucosal surfaces in the body such as those in the gastrointestinal tract or the mucosa of the oral cavity. This mucoadhesive property is beneficial for prolonging the residence time of drug formulations at specific sites, enhancing drug absorption, and improving therapeutic efficacy.
Drug Delivery: Due to its mucoadhesive nature, Polyacrylic Resin E100 is commonly used in the formulation of various drug delivery systems such as buccal tablets, oral films, nasal sprays, vaginal inserts, and ocular formulations. These dosage forms can provide sustained release, controlled release, or targeted delivery of drugs to the desired site of action.
Compatibility: Polyacrylic Resin E100 is compatible with a wide range of active pharmaceutical ingredients (APIs) and excipients commonly used in pharmaceutical formulations. It allows for the formulation of dosage forms with tailored release profiles, improved stability, and enhanced bioavailability of drugs.
pH Sensitivity: Although Polyacrylic Resin E100 itself is not pH-sensitive, its mucoadhesive properties can be influenced by the pH of the surrounding environment. Changes in pH may affect the degree of mucoadhesion, which can be advantageous for specific applications requiring pH-dependent adhesion.
Regulatory Approval: Polyacrylic Resin E100 is widely recognized and approved for use in pharmaceutical formulations by regulatory authorities worldwide. It meets the necessary quality standards and regulatory requirements for pharmaceutical excipients.
Be the first to review this product :-)
Recommend Lab-Service
| Lab Service | Price |
|---|

a cationic copolymer based on dimethylaminoethyl methacrylate, butyl methacrylate, and methyl methacrylate with a ratio of 2:1:1
Tablet Type
Polyacrylic Resin E100 is a cationic copolymer mainly composed of dimethylaminoethyl methacrylate, butyl methacrylate, and methyl methacrylate with a ratio of 2:1:1. This polymer is primarily known for its mucoadhesive properties, making it suitable for various pharmaceutical formulations intended for localized drug delivery.
Here are some key features and applications of Polyacrylic Resin E100:
Mucoadhesion: Polyacrylic Resin E100 exhibits a positive charge at physiological pH, allowing it to adhere strongly to mucosal surfaces in the body such as those in the gastrointestinal tract or the mucosa of the oral cavity. This mucoadhesive property is beneficial for prolonging the residence time of drug formulations at specific sites, enhancing drug absorption, and improving therapeutic efficacy.
Drug Delivery: Due to its mucoadhesive nature, Polyacrylic Resin E100 is commonly used in the formulation of various drug delivery systems such as buccal tablets, oral films, nasal sprays, vaginal inserts, and ocular formulations. These dosage forms can provide sustained release, controlled release, or targeted delivery of drugs to the desired site of action.
Compatibility: Polyacrylic Resin E100 is compatible with a wide range of active pharmaceutical ingredients (APIs) and excipients commonly used in pharmaceutical formulations. It allows for the formulation of dosage forms with tailored release profiles, improved stability, and enhanced bioavailability of drugs.
pH Sensitivity: Although Polyacrylic Resin E100 itself is not pH-sensitive, its mucoadhesive properties can be influenced by the pH of the surrounding environment. Changes in pH may affect the degree of mucoadhesion, which can be advantageous for specific applications requiring pH-dependent adhesion.
Regulatory Approval: Polyacrylic Resin E100 is widely recognized and approved for use in pharmaceutical formulations by regulatory authorities worldwide. It meets the necessary quality standards and regulatory requirements for pharmaceutical excipients.
| Mechanism | - |
| Appearance | - |
| Longevity | - |
| Strength | - |
| Storage | - |
| Shelf Life | - |
| Allergen(s) | - |
| Dosage (Range) | - |
| Recommended Dosage | - |
| Dosage (Per Day) | - |
| Recommended Dosage (Per Day) | - |
| Mix Method | - |
| Heat Resistance | - |
| Stable in pH range | - |
| Solubility | - |
| Product Types | - |
| INCI | - |
Cart
No products